The Special ASEAN Consultative Committee for Standards and Quality (ACCSQ) Meeting on the Issue on Certificate of Pharmaceutical Product (CPP) was held on 23 August, 2021 convened by the ASEAN Secretariat via video conferencing meeting.
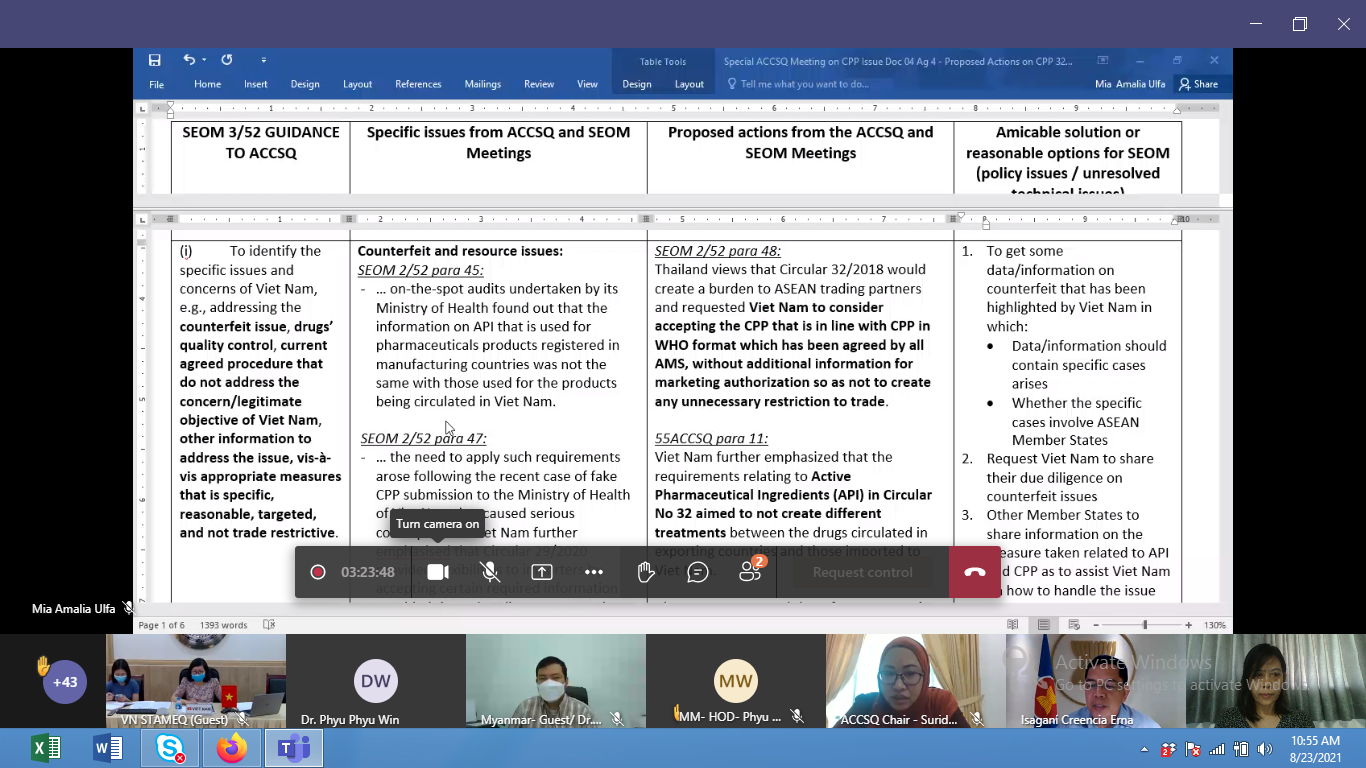
Accordingly, the focal department of ACCSQ, Director General of Research and Innovation and the officials from National Standards and Quality Department (NSQD) together with the relevant authority representatives from Department of Food and Drug Administration (FDA), the Pharmaceutical Product Working Group (PPWG) focal department of ASEAN had also been participated to discuss the Follow up Action Ag Item 4.1 proposed issue of Vietnam on Circular 32/2018 and additional requirements for CPP from 55th ACCSQ Meeting.